
On April 20, 2024, The Expert Consensus on Detection and Clinical Application of Tumor DNA Methylation Markers (hereinafter referred to as "Consensus"), which was condensed by the Expert Committee of Tumor Markers Professional Committee of the Chinese Anti-Cancer Association after six months from outline writing, preliminary draft writing, cross-review, preliminary draft revision, editorial review, consensus voting and other procedures, was published in 2024CCTB China Tumor Markers The conference on Tumor Methylation Markers Forum held a consensus conference, and many experts in the field of cancer from all over the country gathered together to witness the release of the consensus. The successful holding of the "Consensus" conference marks that this clinical guidance document, which gathers the latest evidence-based medical evidence and expert wisdom, has officially entered China's practice, writing a new chapter in the detection and clinical application of tumor DNA methylation markers! Among them, Shanghai Epiprobe Biological Technology Co., LTD. (hereinafter referred to as "Epiprobe Biotech") series of whole cancer markers and methylation innovative detection solutions are included in the Expert Consensus on Tumor DNA methylation Marker Detection and Clinical Application, and the application of guide positioning sequencing, The PCDHGB7, HIST1H4F, SIX6 genes screened by GPS technology and Epiprobe TAGMe series detection products based on total cancer markers became the bright spots of this expert consensus.

In order to promote the application of DNA methylation markers in tumor management, improve the understanding of clinical medical technicians and scientific researchers on their importance, and standardize the detection process to ensure the accuracy and reliability of test results, experts and scholars in the field of DNA methylation in tissue examination, pathology, clinical and basic science, Co-authored the Expert Consensus on Detection and Clinical Application of Tumor DNA Methylation Markers. The aim is to provide a set of guiding suggestions on DNA methylation marker detection and its clinical application for clinicians, researchers and testers, promote standardized development in this field, optimize personalized tumor diagnosis and treatment strategies, and provide decision-making basis for early screening and early diagnosis and accurate diagnosis and treatment of patients. The official release of this "Consensus" is an epoch-making important moment, with landmark significance, the consensus condensed the wisdom and effort of dozens of experts, after a large number of literature review and data collection, repeated discussion and modification of the final text and officially published. The consensus was published in the Chinese Journal of Cancer Prevention and Control on April 19, 2024 (Volume 16, Issue 2, April 2024), hoping that the publication of the consensus can provide a powerful weapon for clinical cancer prevention and control practice.
n the tumor screening scenario, consensus mentions the use of DNA methylation markers in single cancer screening and multi-cancer screening respectively. The application of DNA methylation markers in the screening of single cancers -- DNA methylation technology provides an efficient, non-invasive and widely applied screening method for tumor early screening, and realizes the early diagnosis and screening of cervical cancer. DNA methylation technology can also be applied to high-risk shunt for cervical cancer screening, which can perform initial shunt for human papillomavirus (HPV) positive patients. A team from the International Peace Maternal and Child Health Hospital affiliated to Shanghai Jiao Tong University established a prospective study cohort of 3251 HRHPV-positive women. PCDHGB7 gene methylation detection was performed on the remaining samples of HPV examination. PCDHGB7 methylation testing reduced referrals in 62.2% of non-16/18 high-risk HRHPV-positive women, showing superior specificity (92.1% vs 74.9%). Application of DNA methylation markers in multi-cancer screening -- Application of multi-cancer screening based on whole-cancer marker strategy: By applying guide positioning sequencing (GPS) technology to screen high-precision and high-coverage methylation sites of the whole genome DNA, Yu Wenqiang's team has mined whole-cancer markers based on DNA methylation sites. These DNA methylation markers can be used to screen for a variety of cancers, among which the HIST1H4F gene, which was mined through genome-wide DNA methylation data, was abnormally hypermethylated in 17 cancers. PCDHGB7, a whole-cancer marker screened by the Eubiota team, was hypermethylated not only in 17 cancer types in the TCGA database, but also in 13 clinical tumor samples. SIX6 gene showed pervasive hypermethylation in 678 clinical samples of 10 common cancer types, among which, SIX6 gene hypermethylation in the diagnosis of cervical cancer, endometrial cancer and urothelial cancer area under the curve, AUC values of 0.99, 0.94 and 0.93, respectively, indicate that SIX6 hypermethylation is an early event of tumor progression and can be used for early clinical screening of tumors.
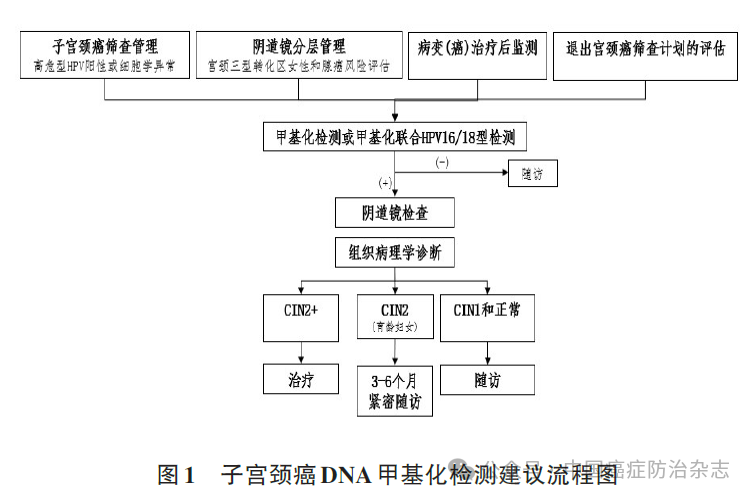
In the context of tumor assisted diagnosis, Consensus mentions the use of DNA methylation markers for exfoliation cytological testing. In gynecological application, DNA methylation detection of cervical exfoliated cells was first proposed as a clinical screening route for cervical cancer and endometrial cancer. Clinical diagnosis of tumor in female reproductive tract: The patency of the lumen of female reproductive tract makes it possible to brush the cells of cervix for non-invasive molecular detection of cervical cancer and endometrial cancer. A team from the International Peace Maternal and Child Health Hospital affiliated to Shanghai Jiao Tong University conducted PCDHGB7 gene methylation detection on the remaining samples of cervical cytology examination. The sensitivity and specificity of PCDHGB7 for detecting ≥ high-grade squamous intraepithelial neoplasia of the cervix were 82.1% and 88.7%, respectively, and the AUC for detecting cervical cancer was 0.97. A large clinical study in China used PCDHGB7 methylation for stratified management of high-risk HPV-positive women, with a diagnostic sensitivity of 82.4% and a specificity of 91.1%. Domestic and foreign studies and experts suggest that clinical pathways for cervical cancer DNA methylation screening are recommended: (1) Stratified management of HPV-positive women with high risk or abnormal cytology; (2) Cervical type III transformation area and potential adenocarcinoma risk assessment; (3) Monitoring of lesions (cancer) after treatment; (4) Evaluation of withdrawal from cervical cancer screening program.
Endometrial cancer cells can be detected in 40% to 50% of patients with endometrial cancer, which makes the use of exfoliated cells for early diagnosis of endometrial cancer methylation clinically feasible. The sensitivity and specificity of PCDHGB7 methylation for the diagnosis of early (stage I) endometrial cancer were 85.71% and 80.60%, respectively. Currently, the test product based on this gene has obtained the EU CE certification. Domestic and foreign studies and experts suggest that clinical pathways for DNA methylation screening of endometrial cancer are recommended: (1) imaging combined methylation screening; (2) Hierarchical management of reproductive tract bleeding; (3) Screening of high-risk groups for endometrial cancer.
DNA methylation detection of exfoliated cells is a new, convenient and non-invasive tool for cancer screening and early diagnosis. Use of shed cell methylation markers in the diagnosis of bladder cancer: Several products based on DNA methylation in urine shed cells for the detection of early bladder cancer have been approved by FDA/CE, such as TAGMe (Epiprobe, Shanghai, China). TAGMe detected the state of SIX6 methylation in urine. The sensitivity and specificity of TAGME for diagnosis of urothelial carcinoma were 86.67% and 90.80%. The product (" TAGMe DNA Methylation Detection Kit (qPCR) for Urothelial Cancer "| PONT-Iqing ®) has received EU CE certification and FDA Breakthrough Medical Device Designation in 2023. HIST1H4F methylation levels in alveolar lavage fluid samples were detected for the diagnosis of lung cancer. The specificity and sensitivity of HIST1H4F methylation levels were 96.7% and 87.0%, and the sensitivity for stage I ~ II lung cancer was 82.6%~84.5%. The PCDHGB7 methylation diagnostic model based on exudated pleural effusion cells was constructed by the team of Yi Pu, and the sensitivity to determine whether lung cancer has pleural metastasis was 94.21%.
In addition, the consensus also mentioned: 1) tumor tracing is an important work in tumor clinical diagnosis and treatment, which can provide a basis for tumor precision treatment. Different from traditional traceability methods, DNA methylation detection based on tissue samples or cfDNA has shown a good application prospect in the field of tumor traceability. 2) MRD analysis based on ctDNA methylation markers does not require tumor tissue sequencing to obtain the characteristic mutation information of the primary tumor, and is not interfered by mutations caused by clonal hematopoietic cloning. It can be used for MRD detection in colorectal cancer and breast cancer patients after surgery or complete remission of other treatments, and is expected to become a key tool for prognostic risk management of cancer patients. 3) DNA methylation markers can be used to evaluate the dynamic monitoring of tumor progression and recurrence of liver cancer, colorectal cancer and prostate cancer, and optimize patient risk stratification and treatment measures. The combination of routine recurrence monitoring markers and DNA methylation prediction models can further improve the prediction efficiency.
Cancer is one of the leading causes of death worldwide, and "early detection, early diagnosis and early treatment" of cancer can reduce mortality rates for all types of cancer. In the future, we look forward to and agree on the "medical" road, continue to expand application scenarios, and further implement and practice tumor DNA methylation marker detection products and services based on total cancer markers, escort public health, and contribute more wisdom and strength to the development of the medical field.
Post time: Apr-26-2024

